How Do Gas Molecules Move Across a Room
Thus the molecules of gas move freely. Energy at high temperatures than low temperatures molecules move more quickly at high temperatures.
The Kinetic Molecular Theory Of Gases
V is the volume of the gas.
. T is the temperature of the gas. How do gas molecules move. The very high speed of gas molecules under normal room.
Explain how an increase in temperature would be expected to affect the rate of diffusion in a gas. If there were no air. So an oxygen molecule travels through the air at 4812 ms which is 1726 kmh much faster than a jetliner can fly and faster than that of most rifle bullets.
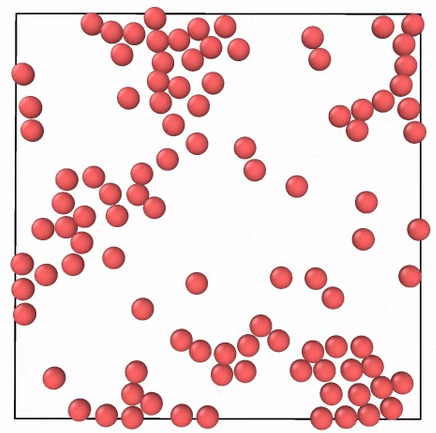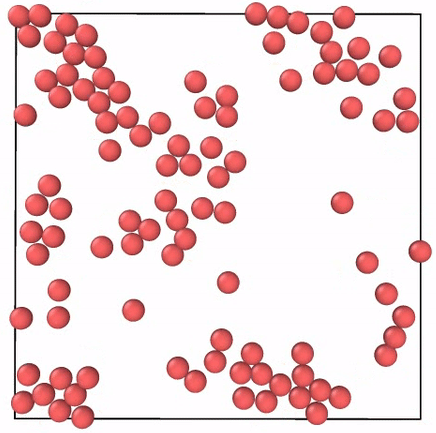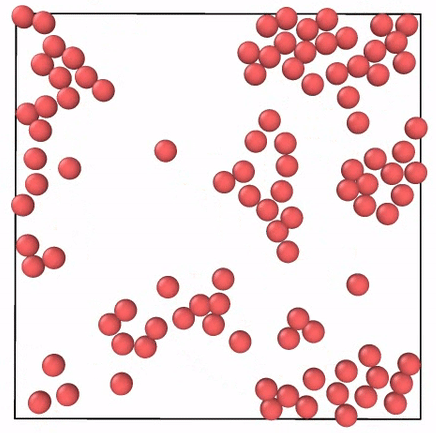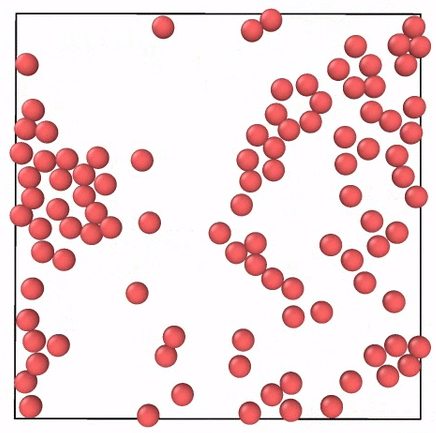
Diffusion is the tendency of the particles of a gas to move from an area of high concentration to an area of low concentration. 5 If gas molecules move so quickly on the order of 1 kilometer per second how come it takes so long to smell a jar of pickles when its opened across the room. When the average kinetic energy of the gas molecules goes up the temperature goes up.
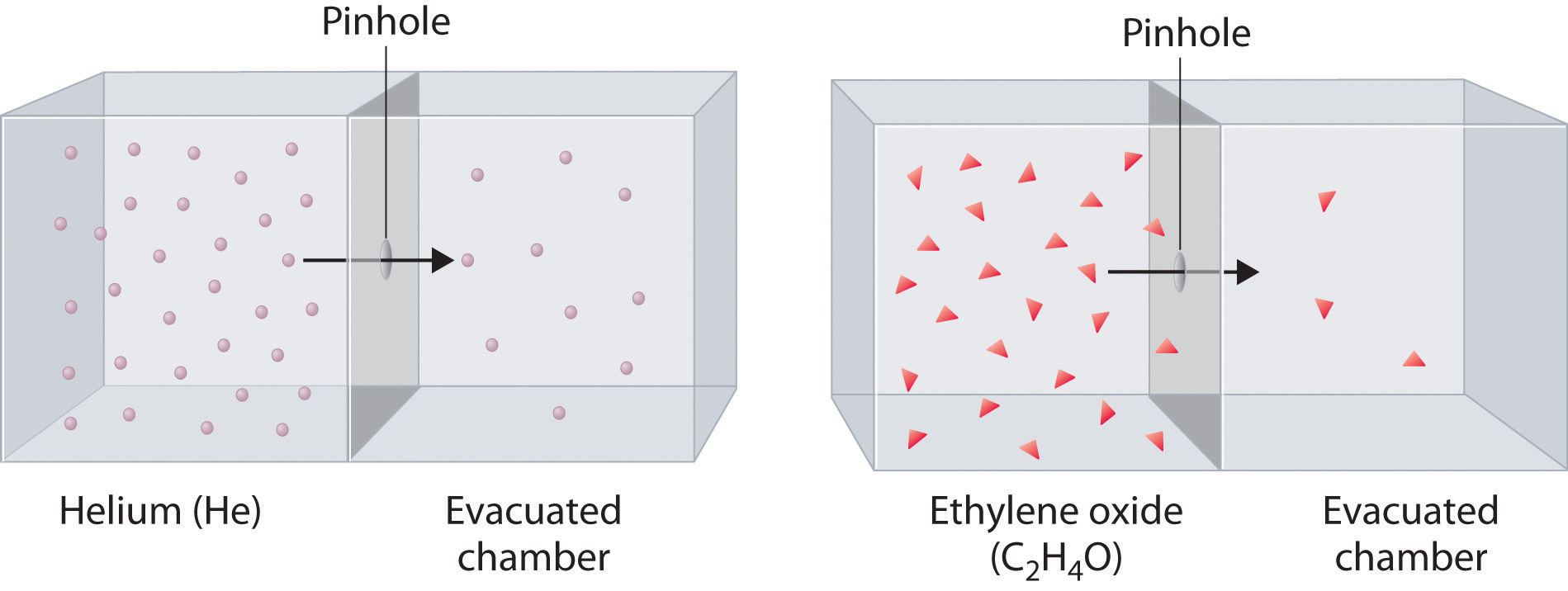
Effusion is the tendency of the particles of a gas to escape through a tiny hole in a. The particles are moving much faster than in a solid. When the barrier is removed and the molecules move into the vacuum they do it with different speeds given by the Maxwell distribution.
Gas molecules dont move in a straight line. As the temperature increases the particles will gain kinetic energy and will move faster the particles will collide and due to this the volume of the gas will increase quicker. Mathematical representation of the law is given in the table.
1 the gas is not overly compressed such that the molecules it is made of do not hit each other very often 2 the molecules do not lose energy when they do hit each other they dont break up or deform 3 modeling their motion on Newtons laws of motion is close enough for us and it is plenty accurate. Effusion is the rate at which a gas escapes through a small hole in a container. Learn vocabulary terms and more with flashcards games and other study tools.
Gases When you add even more energy to the substance you increase the kinetic energy of those particles so much that they lose their state form becoming a gas. In other words diffusion is the tendency of the particles of a gas to spread out and fill a room. R is the universal gas constant 83145 Jmol -1 K -1.
B The cells of the plant would shrink as water from the plant moved into the aquarium. PV nRT NkT. The molecules move all the time with a speed distribution dependent on the temperature of the gas.
Of course at a given temperature lighter molecules always move more quickly than heavier ones. Diffusion is the rate at which a gas travels across a room. Gas molecules can be packed into this space so gasses are compressible.
Because a gas is a large number of very small particles moving very quickly and in random directions the gas will tend to spread out to all the space available to it. Start studying How do molecules move across the cell membrane. So on average they should move into the vacuum with an average speed of that distribution.
Because the KMT states that the amount of kinetic energy is dependent on the temperature the temperature will determine how fast the molecules go in the first place. Now diffusion in simple terms is spreading and mixing of the gas and the faster it is greater the rate of diffusion. N is the number of moles.
C The solution inside each cell would get salty as the saltwater moved into the plant. A The cells of the plant would burst as water from the aquarium moved into the plant. That is very important to understand.
An input of heat is not always accompanied by an increase in temperature however. If the object passes at a low speed typically less than 200 mph the density of the fluid remains constant. Where P is the pressure of the gas.
Another process that involves movement of the particles of a gas is effusion. D The saltwater would not affect the plant in any way. Feb 18 2016.
The temperature of a gas is also important in determining its molecular speed. Attractions keep molecules close together but their energy gives them motion and moves them apart. In a gas particles have vibrational rotational and translational motion allowing them to bounce off of one another.
Gas molecules moves because it is hot and it sperd apart. The heat energy could be used to change the bonding. If we have to calculate the root-mean-square velocity of oxygen molecules at room temperature 25C.
The Temperature of the Gas. How would an increase in atmospheric pressure be expected to affect the rate of diffusion in a gas. In a gas the molecules have a very weak intramolecular bond with each other due to the lack of kinetic energy in the bond.
Gas molecules move around an object as it passes through. Heat goes into a substance the temperature goes up - thats one way it can work. But for high speeds some of the energy of the object goes into compressing the fluid moving the molecules closer together and changing the gas density which alters the amount of the resulting force on.
Both of these phenomena are illustrated by the following figure. If gas molecules are moving at a tremendous speed why does it require several minutes for a scent to diffuse across a room. You can add motion to molecules by giving them more kinetic energy of a possible type such as thermal energy oven fire electromagnetic radiation energy microwave oven or mechanical energy stirring.
Other important behaviors of gases explained by the Kinetic Molecular Theory are effusion and diffusion.

The Behavior Of Gases Chemistry For Non Majors
11 2 Solids Liquids And Gases A Molecular Comparison Chemistry Libretexts

Book 1 Section 5 2 The Kinetic Theory Ppt Download
How Far Apart Are Air Molecules At Standard Temperature And Pressure On Average Quora
What Do Gases Have To Do With Energetic Toddlers By Skanda Vivek Emergent Phenomena Medium

Kinetic Theory Atomic And Molecular Explanation Of Pressure And Temperature Physics

Gases Liquids Solids States Of Matter Kinetic Particle Theory Models Diagrams State Changes Melting Boiling Evaporation Condensing Freezing Solidifying Cooling Curves Particles Pictures Elements Compounds Mixtures Heat Conduction Electrical
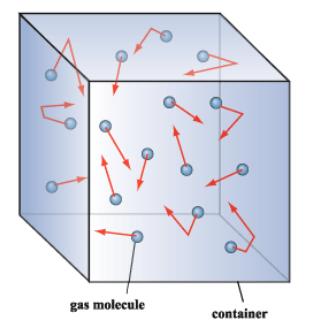
The Fundamentals Of Vacuum Theory
How Are Molecules Arranged In The Three States Of Matter Quora
What Do Gases Have To Do With Energetic Toddlers By Skanda Vivek Emergent Phenomena Medium
How Do Molecules In A Solid Differ From Those In A Liquid Or Gas Quora

What Do Gases Have To Do With Energetic Toddlers By Skanda Vivek Emergent Phenomena Medium
Are Gas Molecules In Constant Random Motion Quora
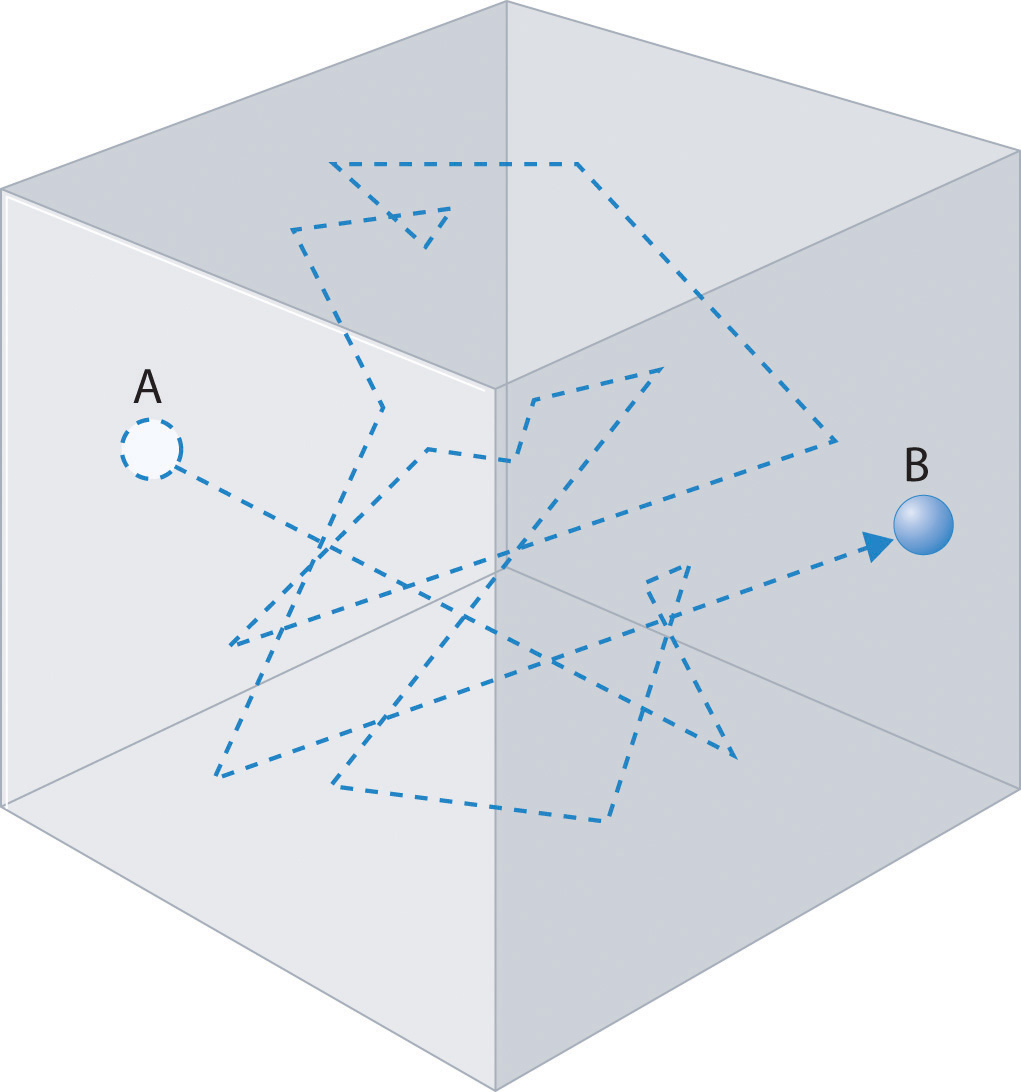
The Kinetic Molecular Theory Of Gases
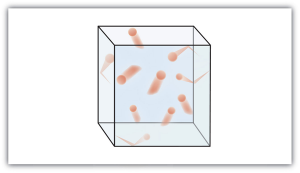
Kinetic Molecular Theory Of Gases Introductory Chemistry 1st Canadian Edition
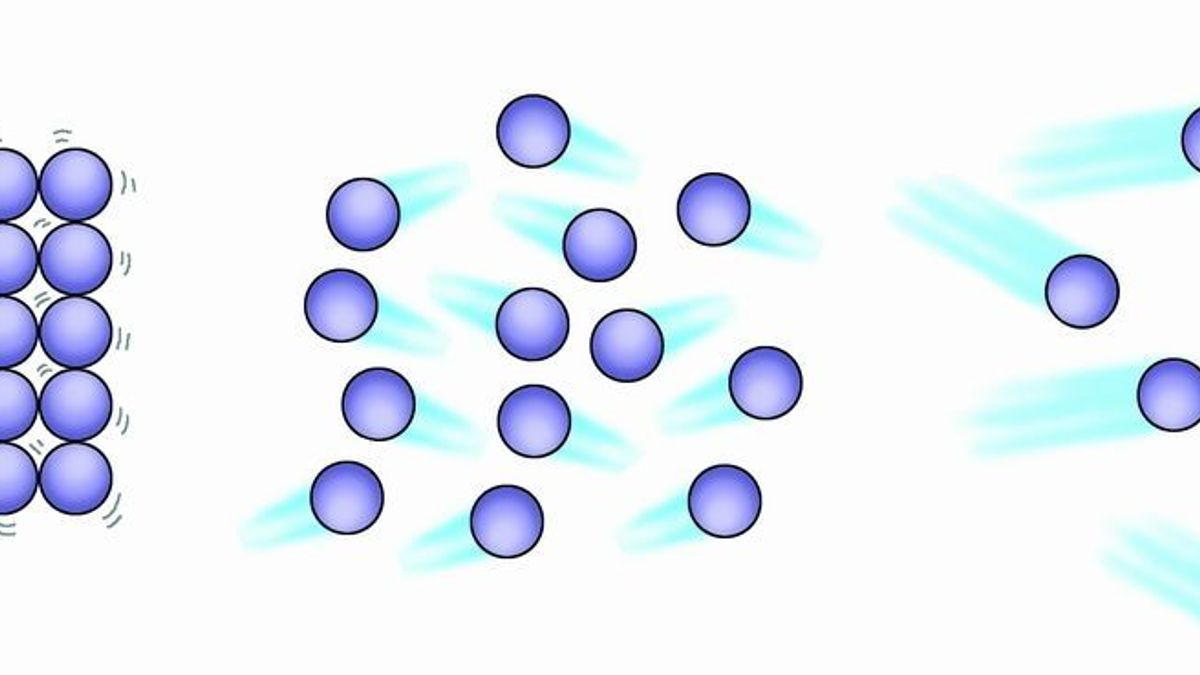
Prove That Particles Of Matter Are Constantly Moving
What Do Gases Have To Do With Energetic Toddlers By Skanda Vivek Emergent Phenomena Medium

Comments
Post a Comment